The UK’s leading development, testing & regulatory laboratory.
Sign up to our newsletter to receive insights and news from ADSL and key technical partners.
Book a free product consultation with our expert team
Book a free product consultation

In the ever-evolving landscape of cosmetic and personal care products, the importance of ensuring safety cannot be overstated. With a vast array of ingredients entering the market, there is greater potential for adverse reactions or health risks prompting a significant focus on toxicological risk assessment.

In the dynamic world of cosmetics, the harmony between a product and its packaging is not just a matter of aesthetics but also of science. This is where compatibility testing plays a crucial role. It’s a process designed to ensure that cosmetic products not only look good in their packaging but also maintain their integrity, functionality, and safety over time.

Non-comedogenic studies refer to scientific investigations conducted to determine whether a product has the potential to clog pores and cause acne or other skin issues.
We are dedicated to setting the benchmark in cosmetic testing through our Objective Assessments and Clinical Trials. Our approach is rooted in a commitment to ensuring product efficacy, safety, and performance, fulfilling a crucial need in the cosmetics industry.

A Product Information File (PIF) stands as one of the foremost legal requirements for introducing a product to the market. Each cosmetic product necessitates its own PIF, encompassing vital information like the Cosmetic Product Safety Report (CPSR), product description, manufacturing methods, and more. This file is subject to scrutiny by the Regulatory authority.
At ADSL Advanced Development and Safety Laboratory, our team of certified toxicologists and regulatory experts is dedicated to assisting cosmetics companies in understanding and adhering to Proposition 65 requirements, ensuring the successful placement of their products in the California market.

Utilising our high-tech equipment in the analytical centre, we are able to offer a wide range of quantitative and qualitative testing solutions. From finished product testing, including heavy metals in pressed powders or solvent detection, to full stability trials for novel product applications, our team will be there to support you through the process

ADSL has been generating CPSRs, both global and region-specific, for over 15 years, and we have the technical expertise and support in-house. This effectively means that we can deliver the final CPSR very efficiently.
Hydration and moisture are pivotal for skin health and aesthetics, and thus stand as central pillars in the development of cosmetic products. Clinical user trials are indispensable in this journey, providing critical data to affirm the efficacy, safety, and user satisfaction of these products.

BS EN 14476 is a European standard that pertains to the evaluation of virucidal activity for chemical disinfectants and antiseptics. This standard outlines the methods and criteria for assessing the effectiveness of these products in inactivating or reducing the infectivity of viruses.
In the realm of cosmetic product development, pH specification testing plays a vital role in crafting formulations that meet precise pH requirements. The pH level of a cosmetic product has a profound impact on its stability, effectiveness, and compatibility with the skin or hair.

In the realm of cosmetic product development, pH specification testing plays a vital role in crafting formulations that meet precise pH requirements. The pH level of a cosmetic product has a profound impact on its stability, effectiveness, and compatibility with the skin or hair.

BS EN 1500 is a European standard that specifies a test method and requirements for the evaluation of the efficacy of chemical disinfectants and antiseptics used in the field of human hygiene.

We are excited to announce that ADSL is now able to offer a U.S. MoCRA Responsible Person Representative Service.

Stability testing for cosmetic products is a crucial quality control and safety evaluation process used to assess how a cosmetic product’s physical, chemical, and microbiological properties change over time under various storage conditions. The primary goal of stability testing is to ensure that the product remains safe, effective, and of high quality throughout its intended shelf life.

Its primary purpose is to evaluate the ability of a product’s preservative system to protect against microbial contamination over its intended shelf life and under normal usage conditions.

The rapid expansion of the Vegan cosmetic market has brought to light a critical need for precise testing methods.

Throughout the cosmetic manufacturing process, ensuring the quality of your products is paramount. Contaminated products not only face restrictions on sale but also pose risks such as spoilage, potential health issues, non-compliance with regulations, and increased vulnerability to further contamination over time.

There are five main requirements for placing a cosmetic product on the EU and UK markets.

Water testing plays a crucial role in identifying and mitigating potential health risks associated with Legionella and other contaminants in various water systems. Here are some key areas where water testing services are in demand and where ADSL may be able to help.

BS EN 1650 is a European standard that outlines a test method for the evaluation of fungicidal or yeasticidal activity of chemical disinfectants and antiseptics. The standard provides a structured and standardised approach to assess the effectiveness of products in controlling or eliminating fungal and yeast pathogens.
In recent years, the CBD industry has seen tremendous growth, with various products flooding the market. However, when it comes to CBD extracts and isolates, businesses must follow a strict regulatory process.

In an ever-evolving world of beauty and skincare, consumers are continually seeking effective solutions to combat the visible signs of ageing, particularly fine lines and wrinkles. As a leading clinical product testing company, we at ADSL are dedicated to helping consumers make informed choices by rigorously evaluating cosmetic products formulated to target wrinkles.
In the dynamic world of product formulation, continuous improvement and adaptation are not just beneficial but essential. At ADSL, we specialise in navigating these complexities, offering expert assistance in improving and modifying existing formulations and developments. As products mature and markets evolve, it becomes imperative for businesses to adapt not only to stay competitive but also to meet ever-changing regulatory, market, and consumer demands.

Lab-scale pilot production serves as a critical step in ensuring the successful development and launch of a cosmetic product, helping to mitigate risks, optimize products, and comply with regulatory requirements while facilitating innovation and cost-effectiveness.

Organoleptic testing in cosmetics is vital in creating products that are not only safe and of high quality but also aligned with consumer preferences, which is crucial in the competitive cosmetics market.

At ADSL, our commitment has always been to provide our clients with the most accurate and reliable data when it comes to cosmetic user testing. This is why we have incorporated the renowned DermaScan® technology into our testing arsenal. To find out why this is a game-changer for our clients please visit our website.

Tissue induration, also known as skin hardness or firmness, is an important parameter to consider when analyzing cosmetic user trials. It refers to the degree of firmness or stiffness of the skin in response to a product or treatment.
Biocidal testing is most applicable in environments and industries where controlling microbial growth is critical to maintaining health, safety, and the integrity of products and materials. Here is a more detailed explanation of where biocidal testing is most applicable and the types of products it is most relevant for:

BS EN 13727, a crucial European standard, delineates the methodology for assessing the bactericidal action of antiseptic and disinfectant products in medical settings. This test, which simulates practical conditions, is applicable to a broad spectrum of products, such as those for surface disinfection, hand hygiene, and instrument sterilization.

Elasticity and tissue stiffness are crucial parameters when developing cosmetic products, particularly those targeting aging skin. Elasticity refers to the skin’s ability to return to its original state after being stretched or deformed, whereas tissue stiffness indicates the resistance to deformation. An Elasticity Meter is often used in product development to measure these parameters.

Trans Epidermal Water Loss (TEWL) is a measure of the quantity of water that evaporates from the skin and passes into the surrounding environment. It is an essential indicator of the skin’s barrier function, and thus plays a critical role in cosmetic product development for several reasons.

Water testing in cosmetic products ensures the safety, quality, performance, and longevity of the products while also safeguarding brand reputation and meeting regulatory standards. It is vitally important that the quality of ingredients is monitored, and in most formulations, the main ingredient is water.

In the field of cosmetic product testing, there are several types of Safety Patch Testing that can be conducted depending on the claims and where you plan to market your products.

High-resolution imaging allows for detailed visualization, providing objective evidence of skin condition before and after product usage. This imaging technique helps in capturing images that can be used as visual proof of the effectiveness of cosmetic products.

Microbiology plays a crucial role in ensuring the safety and quality of cosmetic products. Here are some ways in which microbiology impacts the safety and quality of cosmetics:

The significance of 1,4-dioxane and methanol testing in the US cosmetics market is driven by the need to ensure consumer safety, regulatory compliance, increasing consumer awareness, brand reputation, and international market access.

Preservative Efficacy Testing is crucial in cosmetics to safeguard consumer health, extend product shelf life, comply with regulatory requirements, maintain quality standards, and preserve product integrity. It plays a vital role in ensuring the safety and effectiveness of cosmetic products throughout their lifecycle.

MoCRA, comes into effect on December 29, 2023 and signifies a significant revision of the current regulatory framework for cosmetics established by the Food and Drug Administration (FDA). It introduces new provisions for cosmetic products within Chapter VI of the Federal Food, Drug, and Cosmetic Act (FDCA).

SPF testing, or Sun Protection Factor testing, is a type of evaluation performed on sunscreen products to determine their effectiveness in protecting the skin from the harmful effects of ultraviolet (UV) radiation. SPF is a numerical value that indicates the degree of protection a sunscreen offers against UVB rays, which are primarily responsible for causing sunburn and contributing to skin cancer.

Yes, it is possible to find asbestos in talc. Asbestos is a naturally occurring mineral fiber that has been used in various industries due to its heat resistance and insulating properties.

Do you live in the Paignton or greater Torbay area? Are you fascinated by the science behind skincare? Are you seeking a unique opportunity to make money while contributing to groundbreaking cosmetic research? Look no further! Become a Clinical Cosmetic Product Tester and be at the forefront of scientific innovation!
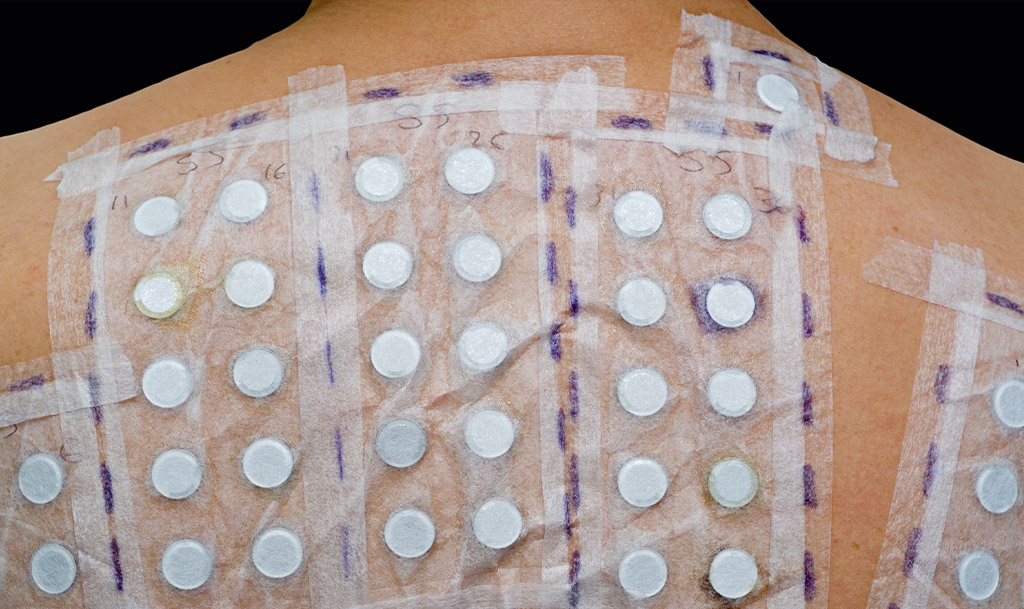
HRIPT stands for Human Repeat Insult Patch Test. It is a type of patch test conducted on human volunteers to evaluate the potential for skin irritation or sensitisation caused by a particular substance.

When you’re thinking mood lighting, candles might be one of the first things that spring to mind. But are they entirely safe? The answer is yes, but only when used correctly.

When designing and developing skincare products, you will want to be certain that the items you are dealing with are safe for consumers to use. This confidence becomes even more important when the ultimate consumer, or end user, is an infant or baby.

Heavy or toxic metals, such as lead, arsenic, cobalt, chromium, mercury and nickel, occur naturally within raw materials used extensively throughout cosmetic products including shampoos, lipstick and eye shadow.

Heavy or toxic metals, such as lead, arsenic, cobalt, chromium, mercury and nickel, occur naturally within raw materials used extensively throughout cosmetic products including shampoos, lipstick and eye shadow.